Cingulate® Technology
Cingulate® Technology
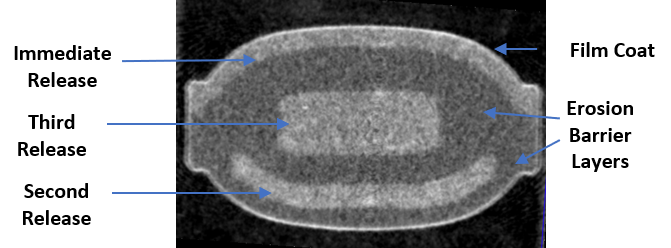
Computed Tomography Scan (CT scan) of CTx-1301 tablet
Cingulate® is developing ADHD product candidates capable of achieving true once-daily dosing using the Company’s innovative Precision Timed Release™ (PTR™) drug delivery platform technology incorporating a proprietary Erosion Barrier Layer (EBL) that provides control of drug release at precise, pre-defined times with no release of drug prior to the intended release.
The technology is an erodible barrier layer that is wrapped around a drug containing core to give a tablet-in-tablet dose form. The barrier layer is designed to erode at a controlled rate until eventually the drug is released from the core tablet.
CTx-1301 (dexmethylphenidate) and CTx-1302 (dextroamphetamine) have pioneering compression technology with specialized release characteristics that may enable patients to take one tablet per day and avoid the use of additional immediate release “boosters.”
We believe our most advanced drug product candidate, CTx-1301, will be the first true once-daily dexmethylphenidate tablet for the treatment of ADHD, providing onset-of-action within 30 minutes and efficacy for the entire active day. CTx-1301 is a trimodal extended-release tablet, based on tablet-in-tablet technology, which provides three releases of dexmethylphenidate hydrochloride at precise times, ratio, and modality of release. Our CTx-1301 release profile is as follows:
- Release #1: An initial immediate-release, or IR, dose providing 35% of the total daily dose beginning within five to six minutes after administration and designed to achieve therapeutic efficacy within 30 minutes; and
- Release #2: Three hours after the administration of the dosage form, the first delayed, sustained release (DR1) provides 45% of the total daily dose released over 90 minutes; and
- Release #3: Seven hours after the administration of the dosage form, a second delayed, immediate release (DR2, the built-in- booster) provides 20% of the total daily dose released over approximately 30 minutes.
In addition, we also believe our second drug product candidate, CTx-1302, will be the first true once-daily dextroamphetamine tablet for the treatment of ADHD, providing onset-of-action within 30 minutes and efficacy for the entire active day.
CTx-1302 is a trimodal extended release tablet, based on tablet-in-tablet technology, that provides three releases of dextroamphetamine at precise times, ratio, and modality of release.
This may give patients the potential for true, once-daily, multi-release tablets. Stimulants are the most commonly prescribed class of medications for ADHD and account for more than 90% of all ADHD medication prescriptions in the United States, where over 70 million stimulant prescriptions were written last year.
With the current ‘once-daily’ extended-release dosage forms, most patients still receive a second or “booster” dose for administration later in the day (typically in the early afternoon) to achieve entire active-day coverage and suffer from a multitude of unwanted side effects as a result. There remains a significant, unmet need within the current treatment paradigm for true once-daily ADHD stimulant medications with lasting duration and a superior side effect profile to better serve the needs of patients throughout their entire active-day.
CTx-1301 (dexmethylphenidate) and CTx-1302 (dextroamphetamine), are being developed for the treatment of ADHD, in the three main patient segments; children, adolescents and adults.
Both product candidates are designed to address the key shortcomings of currently approved stimulant therapies by:
- Providing an immediate onset of action (within 30 minutes)
- Offering ‘entire active-day’ duration
- Eliminating the need for a ‘booster/recovery’ dose of short-acting stimulant medications
- Minimizing or eliminating the rebound/crash symptoms associated with early medication ‘wear-off'
- Providing favorable tolerability with a controlled descent of drug blood levels
With the potential of removing the ‘booster’ dose used by ADHD patients in conjunction with their primary medication, these candidates may provide the important societal and economic benefits of:
- Reducing the abuse and diversion associated with short- acting stimulant medications
- Permitting physicians to prescribe one medication versus two
- Allowing patients to pay for one medication versus two
- Allowing payers to reimburse one medication versus two
Erosion Barrier Layer (EBL) formulation OralogiK ™ is licensed from BDD Pharma
If you are interested in learning more about Cingulate’s proprietary Precision Timed Release™ (PTR™) platform, please contact Thomas Dalton at 913-942-2301 or ir@cingulate.com

